A new minimally invasive device to deliver stem cells to the spinal cord of patients with ALS
J.Patrick Johnson MD, Pablo Avalos MD, Doniel Drazin MD, Harish Babu MD, Chirag Patil MD, Peggy Allred DPT, Richard Lewis MD, Robert Baloh MD, Clive Svendsen PhD | Regenerative Medicine Institute, Spine Center, Department of Neurosurgery, Department Neurology, Cedars Sinai Medical Center, Los Angeles, CA USA
Study Overview: Injection of human neural progenitor cells secreting Glial Cell Line-Derived Neurotrophic Factor (GDNF) for the treatment of Amyotrophic Lateral Sclerosis
Hypothesis: Preclinical studies suggest that GDNF-secreting neural progenitor cells injected into the spinal cord could provide essential trophic support to sick motor neurons and may limit the progression of degeneration in ALS patients
Procedure: A device/system for delivery of GDNF-secreting neural progenitor cells is needed to treat patients with ALS and is presented here
ALS Stem Cell Injection Procedure
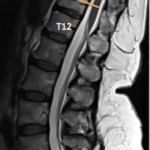
- Lower thoracic laminectomy overlying the caudal spinal cord at the L3-5 spinal cord level
- Spinal level is determined from anatomic studies and correlating MRI imaging of the conus and surgical/vertebral levels range from T10 to L1
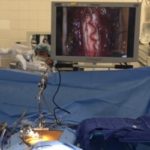
- Delivery of stem cells is through a proprietary stereotactic device and micro-injection system that is mounted on a commercially available surgical retractor
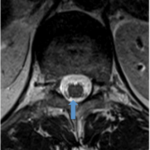
- Injection site is 1mm medial to DREZ at depth into dorsal aspect of ventral horn cells
Results
- Unilateral Injection of stem cells into the spinal cord at 10 sites with 2mm intervals
- 11 patients with ALS have been treated so far in this Phase 1/2a non-randomized study, and no significant adverse events related to the device have been seen.
- Short-term transient neuropathic pain in the lower extremity is seen.
- Post-operative imaging has not demonstrated any changes in the spinal cord parenchyma.
Conclusion
- Precision delivery of stem cells into the spinal cord with a new minimally invasive device appears to be feasible based upon early results of this study.
- The last patient (18) will be treated July 2018.
- This Phase 1/2a study will be completed mid-2019 and final results will be reported.
- At this early stage, technical and safety goals associated with this minimally invasive investigational device appear to be attainable.